Physics has always been an interesting topic, but it was during a span of only 50 years that physics completely changed the world. The movie “Oppenheimer” showed in great detail the history of what happened and how they got to the atomic bomb. But this is only part of the story. This revolution, that ended with the bomb in 1945, started already in 1895, and it is what I will try to explain here.
1. Introduction
Physics is a discipline that goes back to antiquity (600 BC). It start from Greek philosophers that considered physics as the understanding of nature; what is natural, or what is the normal course of things. This disciple didn’t change for 2000 years, until 1687, and Isaac Newton was the most important person in this revolution. Physics became the science of phenomena of the natural world. The job of physics was to describe phenomena and try to link them together in a theoretical overview, without talking about the essence of things. Essentially, it became the discipline of experiments, which was unthinkeable for the ancient Greeks, as it no longer is the natural behaviour, but you force nature to see what happens with experiments. Mechanics is the basis of modern physics and it follows the definition very well. This new definition is narrower and it is still what is used in today’s physics as well. After Newton, science became important, and with the era of enlightenedment, science became part of university education in the 1800’s.
The big revolution of physics was in the beginning of the 20th century. So in the 1950s most important theories were already done, and these physicists moved on to other disciplines, giving rise to new discoveries (Watson and Crick, 1953 with the double helix DNA), thanks to the different approach.
In the 19th century physics was science of particles and fundamentals. The most important science was Chemistry, since you could work in the industry. Most students went into chemistry, as you can get lucrative discoveries and there was lots of work. Pure physics was a basic science, about the laws of mechanics and heat. Even Max Planck’s professor adviced him not to go into physics. Then in the beginning of 1900s, with Einstein and Quantum Mechanics (QM) and Relativiy, it became the most important science. Suddenly physics became a central discipline that introduced new discoveries and new laws. Now the chemists had to learn about physics, about quantum chemistry. The biologists went to physics as well to understand about molecular biology; and everyone was admired and inspired by it. This type of physics, also introduced a new way of thinking. QM and relativity redefined science. Is science describing nature? No, it is just a description of what you see in nature; it just explains the phenomena and experiments. There is a separation between scientific theory and relaity. Theory may give a description of it, but it is not the real thing.
This revolution happened in the span of only 50 years. In 1895 we have the first Nobel prize for physics. In 1905 Einstein, who was a lot younger than the other physicists published 3 important papers. They had the fifth Solvay conference in 1927, where they discussed QM (that was explained with waves). And in the 1930s, nuclear phycics became the new standard, as everything was said about QM. It was in 1933 that the nazi’s came to power, banning Jews from all official positions, and many jobless physicists spread out to all over the world, of which many went to the USA.
But the whole revolution came to a halt with the creation of the atomic bomb (Manhattan project), which started in 1942. It was so expensive and impressive, but on the other hand also very dangerous. Governments began to implement science policies, as the money had to be paid by the taxpayers. These policies defined a new view of physics, it was no longer free of investigation, but guided by money and society. It also shifted from just a single person to large teams to investigate something. Physicists now had to write funding proposals to fund their project.
Three different revolutions due to physics in the 1900s
- Conceptual revolution: Ohysics became a new field with new phenomena that needed to be studied without empirical data. Previously, most people went for experiments, but now more and more theoretical physicists came. QM and and relativity were also conceptually new. It changed the way people looked at the reality.
- Professional revolution: The identity of a physicists changed. Before this time, scientists were like philosphers who might do some experiment. You could think of an experiment in the morning and finish it in the afternoon. Yuo were able to publish whatever you want, without peer review. Nowadays, it takes years and you need the right equipment. With the Manhattan project, we saw that it was a large team, with scientists fro all over the world who migrated to the USA. Physicists in the USA were different from their European counterparts. In the USA it was much more practical (they knew how to gain money from it), compared to the philosophers in Europe.
- Social revolution: A change from fundamental physics with electrical and opical phenomena, to radar technology and the atomic bomb in the second world war. Science became a matter of social debate, not just about funding, but also whether it is beneficial to society. Anti-nuclear movements had impact on politicians, and they in turn had impact on scientists. If the public funding stops, so does physics, as it started to cost a lot of money.
2. Classical Physics
Boltzmann was the one who first coined the term “Classical Physics” in 1899, when he wrote:
“The goal of physics was for all times reduced to establish the force laws between two atoms, and then to solve the equations of these interactions under the relevant conditions. How all of this has changed. I am the only one left who still embraced the old doctrines with unreserved enthusiasm. I therefore present myself to you as a reactionary, one who has stayed behind and remains enthusiastic for the old classical doctrines as against the men of today.”
That was his own understanding of Newton’s definition. Everything must be reduced to interactions between particles and that should be the final aim of physics. As it was about evolution with only the strong ones will remain, and the poor ones will tay behind. Boltzmann didnt feel accepted by the other physicists, as everyone else was thinking about a revolution that he didn’t like, he eventually comitted suicide.

But it has always been sort of an evolution; Newton was also reacting against whatever was before him. For him, everything was made up of particles, even photons and matter. Laplace is the one who revived Newtonian physics, as most of what we now know from Newtonian physics, is formulated by Laplace. Some of his famous writings are “Exposition du systeme du monde (1796), Mecanique Celeste (1799) and Theorie analytique des probabilites (1812). It was in the latter one he write:
“Some perfect genius who knows the location and movement of all the particles in the world, can preduct all futures (and past) events.”
Classical physics has three characteristics:
- Corpuscular mechanics: Meaning small particles.
- Mathematical formalism: It should be accurately explained in mathematical formulae.
- Causality: No random phenomena. Everything can be explained by the preceding conditions of the world.
As a result of Laplace’s work, the French were very well versed in mathematics, such as Coulomb, Fresnel, Biot, Ampere, Fourier, Poisson, Carnot. Also many people worked on optics, with interference and polarization.
On the other side of the border, you had germany, who just lost the war against France and they tried to catch up with promoting philosophy studies at the highest level of university (PhD). Physics and mathematics were considered part of philosphy, it became more important in a deeper sense.
During this time, there were two main problems that people tried to solve. The first one was the problem of matter. Very often problems were divided into ponderabilia (with mass) and imponderabilia (without mass, such as het light electricity). You had Faraday who, without having any education, was working with the concept of magnetism. He had concepts of lines of force, but could explain what those lines of force were. Ostwald had another direction, as he was trying to do chemistry on a physics base. He noticed there is heat in chemical reactions, and he wanted to explain all of nature as a function of energy. We also have the famous dutch physicist Lorentz, who was trying to make aether theories to both ponderabilia and imponderabilia. However, no one knew what this aether really was.
The second problem was unification of theories. On one hand you had theories on radiation and optics, on the other hand you had particles. It was almost embarrashing that you had 2 disciplines that describe everything, but they could be discussed completely independent from each other. Maxwell had some success, as he was able to unify electricity, magnetism and optics in one theory, “Treatise on Electricity and Magnetism (1873)“. This was also the problem that Boltzmann was facing. Everyone else seemed to go in the direction of aether, which is something difficult to understand. He was saynig that the physicists were giving away their idea of particles and replacing it with something which had no empirical data at all.
Since the problems accumulated durnig the 19th century, people were looking for inspiration to solve he problem. The atmosphere of the time was neo-romanticism, which means that there are things in the world beyond your understanding. Maybe “matter” was not the ultimate reality. They believed that their science was not enough to understand the world, and they had to look for new ideas or concepts that could explain what is beyond. The older generation of scientists were not really prepared for new laws, however the young people were. One such physicist was inspired by this neo-romanticism. He was looking for concepts that were completely unknown before, and his name was Albert Einstein. [Helge Krahg, Higher specilations, 2015)].
3. Quantum revolution

At the end of the 19th century, there were approximately 1000 physicist all around the world. Within this tight community, Germany was the leading nation from around 1860. Not just in physics, but also other sciences, this continued to be the case until 1930. Even though they not had as much money as the British physicists, the German community was more productive than any other. German scientists have always been very organized, which may be the reason for their success. Until 1925, almost half of the citations were German papers. The end of the 19th century, and in the beginning of the 20th, many discoveries were many in a short period of time; it was like every year a new research field was discovered, that again produced new discoveries. [Richard Staley, Einstein’s Generation (2008)]. Some examples are:
X-rays
W. Rontgen worked on cathode rays at home that produced imaging. He published his paper in 1986, including a picture of the hand of his wife. Within a few months, people came with applications, which was a great success, until they found that X-rays damage the human body.
radioactivity
Henri Becquere was studying Radium salt, and by accident he saw on a photographic paper the capture of a medallion that he kept in the office. He discovred that Radium send some kind of waves, and that it was radioactive.
Plum pudding model
J. Thomson discovered particles with electrical charge, as was already theoretically predicted. He came up with an atomic model that involved Aether (positive charge) with small charged particles (negative charge) moving around like in a soup. It is different than our current model.
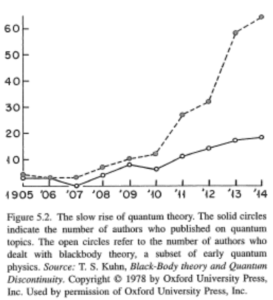
One of the major problems was the Blackbody radiation, which was first defined by Kirchhoff, who shown that it only depends on temperature. Several people tried coming up with equations. Boltzmann’s law with $T^4$ dependency, after that we have the Wien’s law (also called Displacement law). Max Planck found a way to derive the Wien displacement law, but it was not enough, as it did not hold for all frequencies. He restarted and used a quantum hypothesis, so the energy levels of the oscillators were on different levels. He had actually hoped that the concept of discrete levels would go away, through some limit, but it didnt’t. He found a formula that looked right, but without a theoretical foundation on why those levels exist, and nothing in between. Planck hoped it was not a revolution, he wished he could explain it by classical mechanics, but most other physicists were happy, as they had an equation they could work with. There was only 1 person who realized the radical, nonclassical nature of Planck’s theory, but at this time he was not a physicist yet.
3.1 Einstein

Einstein worked after graduation in a patent office, so he knew about all the experimental physics from the patents he saw. He was a young man without a career in physics, so he had a different approach for it, and he didn’t care about his image if he was wrong (as he was not well known at this time). He truly came onto the scene in 1905, when he published 4 major papers. One of them was about the quantum hypothesis, a new approach on the nature of light. He believed that light consisted of quanta, referring to the photoelectric effect as proof, which fitted with his theory. But this effect was already well known, therefore it wasn’t really interesting for the physics community. This new theory wasn’t noticed by others, as it was nothing new for experimental physicists. He was known for publishing 4 papers in one year, but nothing particularly in need of attention. Hence the revolution did not start here. The breakthrough came in 1907 when he applied quantum theory to specific heat, which was researched by chemists. There were many people studying it, and they could use his theory, especially for low temperatures. Because this was a field with lots of research, the number of papers on quantum theory increased rapidly, and it became a major research topic.
3.2 Solvay conference (1911)
In 1911, the first Solvay conference took place in Brussels. Solvay was a chemistry industrial, who created artificial soda. The founder of the company had a very deep admiration for science, so he gave money to science and wanted to support it. He created the conference. The idea of the conference was that only the best of the best and leaders of the field should come together. These conferences were on physics and chemistry. He wrote to Nernst (a chemist), who was working on thermodynamics, to know who were the “best” to invite. As Nernst was working on specific heat, he was fully aware of the new quantum theory, and he invited some of the major scientists at that time. The conference was about specific heat theory, not about quantum theory, but the discussion went that direction anywyas. Nernst invited 50% experimental physicists and 50% of theoretical. While in fact only 1% of the world did theoretical physics, and any other ordinary conference would be 99% filled with experimentalists. [Helge Krahg, Higher specilations, 2015)].
Because this was the first conference with such high amount of theoretical people, they were able to interact and come up with new concepts, while the experimentalists could think of ways to solve the problems with expierments. Several people received Nobel prizes, and many more of them would receive them later on. This is the moment Einstein started to gain popularity. [Jungnickel and R. McCormmach, Intellectual Mastery of Nature, vol. 2 (1986)].
3.3 Copenhagen
Relativity theory was different from quantum theory in that it captured the heart of the masses and philosphers, as it had a lot to do with paradoxes. QM on the other hand was more technical and more confined with specialists. In the beginning not many understood it, and it was not always accepted as something revolutionary. There were always people hoping it would go away, or that it could be replaced by classical physics. The point of no return in QM would be the Copenhagen interpretation (1927). Einstein was the leading physicists between 1905 and 1925, but he was more of a loner, not a typical professor with students. On the other hand you have Niels Bohr, who was a typical physicist of this period. He was Danish, and was with Einstein part of the younger generation. He was partly Jewish, which had its own advantages and disadvantages.
At a very young age, he published a paper on electron theory, and received funding to go to the UK for an internship, working for Thomson and Rutherford. When he returned to Copenhagen, he wrote a paper on the atomic mode. Rutherford had an atomic model comprosed by a limited number of electrons circling around a positive nucleus, but his model was not stable. Bohr was trying to reconcile electromagnetism in this model, and he made 3 postulates, to make his model fit. These postulates had no real reason or argument on where they came from, but they were inspired by quantum theory.
- The electron is able to revolve in certain stable orbits around the nucleus without radiating any energy, contrary to what classical electromagnetism suggests. These stable orbits are called stationary orbits and are attained at certain discrete distances from the nucleus. The electron cannot have any other orbit in between the discrete ones.
- The stationary orbits are attained at distances for which the angular momentum of the revolving electron is an integer multiple of the reduced Planck constant.
- Electrons can only gain and lose energy by jumping from one allowed orbit to another, absorbing or emitting electromagnetic radiation with a frequency v determined by the energy difference of the levels according to the Planck relation: ΔE = hv, where h is Planck’s constant.
The strength of Bohr’s theory was not in its theoretical foundation, but its experimental confirmation, over a wide range of phenomena. The Balmer and Lyman series could explain why you had to subtract 2 numbers, as it was the subtraction between 2 energy levels. The red line in the hydrogen spectrum has a double structure. Bohr was unaware of it, but it turned into another confirmation, but it required a major extension of the theory, made by A. Sommerfeld. Bohr did not only publish his paper based on H en He, but he also tried to apply his model to all atoms, but it was not so successful. He did try to explain why the table of elements was the way it was. Since Mendelejev, the table was just made pure on the resemblance of properties of elements. In this time, that was okay. But in the 1900s, chemists were less convinced that it based on this alone, and they could make other tables, that were based on grouping. The Bohr model could explain the table’s structure, with the position of electrons. The more electrons there are, the more layers there are. Bohr then published the first physical interpretation of the Mendelejev table, that shwoed that the position of elements was based on atomic structure.
After the first world war (WW1), physics started to rise again. A lot of interest went to Einstein and his relativity theory, but in Copenhagen Bohr was able to fund his institute of theoretical physics. He became a host of quite a lot of physicists, that went to Copenhagen because of the easy-going nature of Bohr. He was always open for discussion and he would debate with anyone. At the same time, Germany had financial difficulties, and it was being boycotted because of the war. German scientists could not collaborate with sciencists from Allied forces. For many years this continued, but it didnt work out completely. Several countries stayed neutral during WW1, such as The Netherlands, Switzerland, and Denmark. The Copenhagen institute was a way to meet german physicists. As example, Heisenberg was the assistant of Bohr in 1925. A lot of the people in Copenhagen were not students, but well-established physicists that went there to work and discuss with Bohr.
Of course he was able to attract younger students as well. So many of these physicists that were responsible for QM were young. That is why Pauli refered to QM as “Knabenphysik” (Boys physics). Nobody of them had a PhD yet, and were still at the beginning of their career and working on topics that were on the forefront of science. Many of these bright young physicists felt, arrogantly, that QM belonged to them and that most elder physicists were just incapable of mastering the theory. They thought that they could do it better than the preceding generation, and tried to make the theory very difficult, so that the older generation would not be able to master it anymore. An example of the older generation is Einstein (around 50 years old now), who did not understand anymore what was going on with GM.
3.3.1. Heisenberg & Schrodering
Heisenberg started his research based on the research of Einstein, who had developed his relativity theory based on descriptionism attitude: “The physicist does not have to present a model of reality, but describe the observation”. Einstein introduced 4D reality, but never claimed that reality is 4D, he just needed 4D to explain his relativity theory. Heisenberg wanted to treat QM with just observables. He created a model based on matrices. Many physicists wre skeptical because of the theory’s lack of visualisability and its unfamilair mathematical formalism
On the other hand you have Schrondering, who was already somewhat older. he attempted QM with a different approach, namely with one based on waves. He went even as far as to prove that his wave mechanics, and heisenberg’s matrix mechanics were equivalent.
Back then, most physicists were experimentalists and didnt know much about matrix mechanics, so Schrodinger’s equation had great advantages, and therefore muche easier to use. In the end, they took Schrodinger’s side, instead of the heisenberg’s model.
Then Heiseberg came to a second big event. he shwoed that no matter how accurate the instrument used; QM limits the precision when two properties are measured at the same time. He concluded that to make a measurement at such small dimensions, you must use some kind of radiation with high energy. Therefore, the act of measurement itself would impart so much energy to the atom that it would cause significant disturbance or disruption. So you can either make a determination of momentum or a determination of position, but never at the same time.
This understanding lead heisenberg to think about causaility that was proposed by Laplace. The assumption that you can see the future and past by knowing the position and momentum of all particles was wrong, because you cannot know both. If you determine one observable in extreme degree of precision, nature will prevent you from knowing the other. Nature will interfere in a sense. Heisenberg discovered the uncertainty relations. Bohr understood that uncertainty principle had to do with the duality of matter as particles or as waves). It was complementarity, and a physicist has to know when to use a particle or when to use a wave. This lead to the Copenhagen interpretation, which is a statistical interpretation of wave mechanics, with the uncertainty equation and the complementarity of particles and waves.
This was the end of the evolution of quantum mechanics. People moved on to a new field of interest, namely nuclear physics
4. Nuclear Physics
Enrico Fermi, an Italian physicist, recognized by his peers as a genius. He was born in Rome, at a young age, he obtained his PhD and travelled to Germany and the Netherlands. When he returned to Rome, he became a professor of theoretical physics (the first in Italy). He married a Jewish girl, and with his work, he succeded in making Rome one of the important places in European physics. He won the Nobel prize, but did not return to Italy after collecting his Nobel prize in Sweden. He immediately moved to the USA, because of the fascist regime and the anti-Jew laws that were introduced in Italy. Fermi was one of the few European physicist that had a good career in the USA, as most others were not accepted in a positive way (they were foreigners, communist view, who did not speak English well).
4.1 The discovery of the neutron
The discovery of the neutron was a very important step in understanding the nucleus. It was very difficult to detect, and at at first there wasnt any need for it. Scientists only dealth with protons (+) and electrons (-). However, Fermi noted that some things dont add up very well. Some scientists envisioned there were additional protons in the nucleus, but than the total net charge would not be zero. The discovery of the neutron happened in 1932, when experiments were done by bombarding Beryllium wth alpha particles from a Polonium source. Some scientists thought this highly penetrating radiation consisted of high energy photons. But how can this kind of radiation have so much energy to kick out these heavy protons? Chadwick found one solution; the radiation was not consisting of photons, but of neutral heavy particles, with a mass similar to a proton. He did not think there would be an application for it, as these particles were neutral. However, Szilard had another idea; if neutrons could be pushed out from one nucleus, it can be used to bombard another nucleus, and fission can be induced, which releases an incredible amount of energy. This made nuclear weapons possible. [Niels Borh and John Archibald Wheeler, The Mechanics of Nuclear Fission, (1939)]
Nuclear fission marks a pivotal moment in the history of nuclear physics, but its roots lie in earlier discoveries and theories. With Chadwick’s discovery of the neutron, it opened new pathways for understanding atomic structure and nuclear interactions. At the Solvay Conference of 1933, while beta decay wasn’t the central focus, it was a topic of discussion among physicists.
Fermi played a significant role in advancing the understanding of beta decay. He proposed a theory in 1933 that described beta decay as a process in which a neutron in the nucleus transforms into a proton, releasing energy that manifests as an electron (beta particle) and a neutrino (Italian for little neutron). This theory introduced the concept of weak nuclear interaction, a fundamental force distinct from gravity and electromagnetism.
Fermi’s work extended beyond theory; he recognized the potential of neutrons as tools for inducing nuclear reactions. By bombarding nuclei with slow neutrons, Fermi and his team increased the likelihood of interaction. In 1938, Fermi’s research took a significant turn when it became clear that the ‘trans-uranium elements’ he and his team had been seeking were not present in their samples. This realization paved the way for further investigations, eventually leading to the discovery of nuclear fission later that year by Hahn and Strassmann. When the idea of splitting the atom, to produce energy and neutrons was published, many people understood that it was a dangerous discovery, especially with what was happening in europe at the time. Most of the physicists stopped publishing about nuclear physics during the way. In the end of 1945-46 a massive amount of papers were published regarding the discoveries during WW2.
4.2 The second World War
During the second world war, research was only possible in the UK and USA, as the americans were not interested in being involved in the war initially. Szilard tried to convince not only physicists, but also politicians to do something about it. He even went to Einstein, who was well familair with the belgium royals (as the best uranium was found in the Belgian colony, the Congo). It took until 1941, with the attack on pearl harbor, that the USA entered the war.
The Manhattan project started, and a metallurgical laboratory was founded in Chicago where Fermi worked. It was a military project, and at the end of the war, tens of thousands of people were employed. The civilian leader of this project was Oppenheimer, a theoretical physicist. One of the important steps was tomake a reactor, to determine if the chain reaction was possible. This was the work of Fermi. Several universities were involved in this research, but the central place was in a new site, completely isolated from the world. This village was called Los Alamos. It grew in population, up to 2000, all relatively young people. The sucessful test of the plutonium bomb occured on July 16, 1945 at the so-called Trinity test side in the New Mexico desert.
When the war finished on in Europe, the Japanese were still attacking. On August 6, 1945, the uranium bomb was dropped on the Japanese port city of Hiroshima. Three days later, the plutonium bomb destroyed Nagasaki. Almost 200,000 people died in those two blasts. The American public’s attitude was anger and suspician against the scientist who contributed on the atomic bomb. In only a few years after dropping the bomb, the view of scientists went from a figure of stature and wisdom to an enemy. [G.S.Allen, Master Mechanics & Evil Wizards, (1992)].

4.3 The aftermath
Lots has changed during this period. Projects became larger and larger. We’ve gotten space research, big machines were build, like the cyclotrons in Berkeley. And importantly, the USA became a scientific superpower, which was mainly due to money. Previously, most universities were independent institutes. Robert Millikan introduced a strategy to concentrate money on just a few research universities (top 10). This money came from philantropists, such as the Rockefeller foundation. Among these universities were Caltech, Harvard, Princeton, MIT, etc. Instead of students going to europe to do their PhDs there, now people came to the USA to study there.
During this time, Europe was recovering from the war .They were in need of money and support. The rebuilding was founded by the Americans, however they did it with the intention to know what was happening in Europe. CERN is such an example. It was an european high energy institute that collaborated with Ammericans, so they could see the state of knowledge in Europe, and make sure that physicists don’t go to Russia. Unfortunately, not much has changed since then. I truly hope that we can learn from the past and make better choices for the future.
5. Links & Suggested reading
- Emilio Segré, From Falling Bodies to Radio Waves. Classical Physicists and Their Discoveries (1984)
- Helge Kragh, The Quantum Generations. A History of Physics in the Twentieth Century (1999)
- David C. Cassidy, A Short History of Physics in the American Century (2011)
- Jon Agar, Science in the Twentieth Century and Beyond (2012)