The Physics and Technology of Metals
Understanding the conductivity of metals involves exploring their high electron mobility, Ohm’s law, and quantum mechanical models like Drude and Sommerfeld. This article discusses how metals conduct electricity, the role of energy bands, and how electron interactions shape conductivity, highlighting key concepts like drift velocity, Fermi energy, and relaxation time.
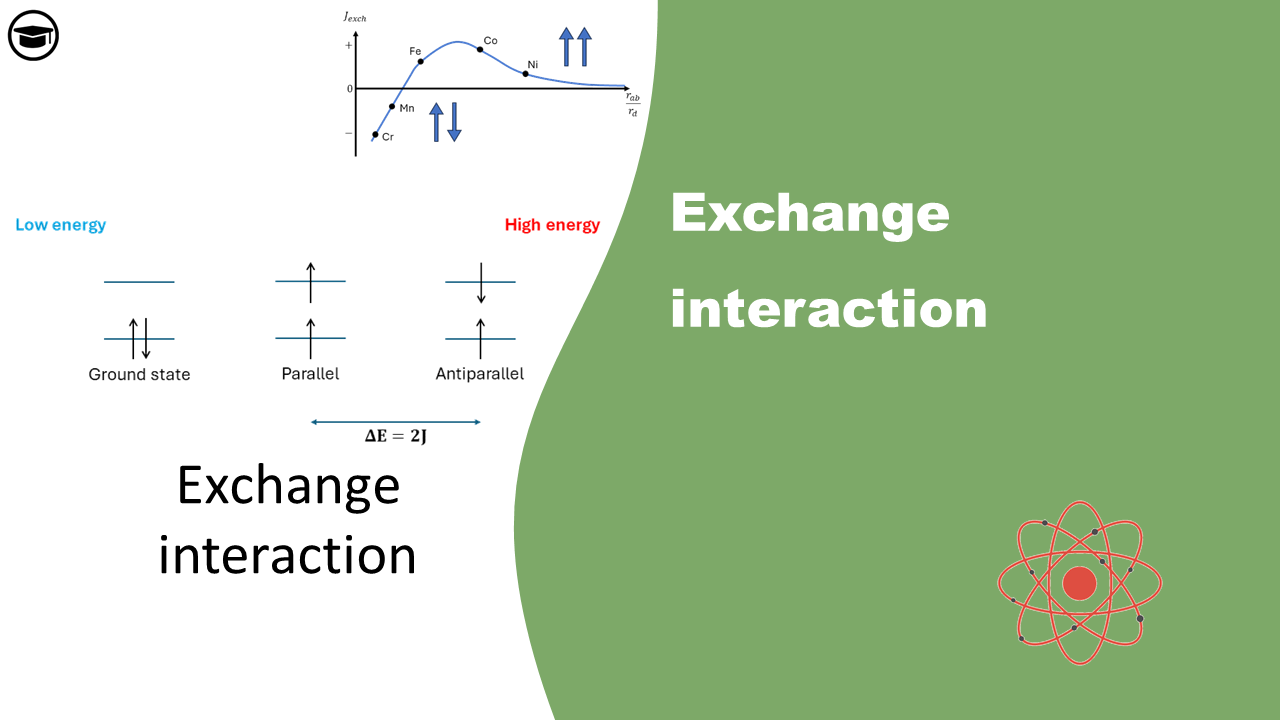
Exchange interaction
Electrons prefer parallel spins due to the quantum mechanical concept of exchange energy, which lowers their system's total energy. This preference is a result of the Pauli exclusion principle and the antisymmetric nature of fermion wavefunctions, reducing Coulomb repulsion and stabilizing the system in quantum mechanical interactions.
The Bohr atom model
The Bohr model revolutionized our understanding of the atom. It proposed electrons exist in fixed energy levels, challenging classical physics. This explained the hydrogen spectrum and laid the foundation for quantum mechanics.