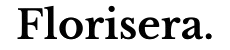Exchange interaction
Electrons prefer parallel spins due to the quantum mechanical concept of exchange energy, which lowers their system's total energy. This preference is a result of the Pauli exclusion principle and the antisymmetric nature of fermion wavefunctions, reducing Coulomb repulsion and stabilizing the system in quantum mechanical interactions.